New ATRP Approach Could Transform the Manufacturing of Specialty Plastics
The novel method expands the scope of polymers that can be created using emulsion polymerization
Media Inquiries
Researchers in Carnegie Mellon University’s Department of Chemistry(opens in new window) have improved a popular technology used to generate a range of industrial plastics for applications ranging from paints and coatings to adhesives and sealants. Using environmentally friendly reaction conditions, including running the reaction in water with light and a water-soluble dye, the novel method offers a promising approach for creating polymers in a greener and more practical way during the emulsion polymerization process.
In a commercial setting, emulsion polymerization is one of the least expensive ways of making a range of useful polymers. The process is limited, however, because scientists cannot precisely control the molecular weight and dispersity of the resulting polymers. And controlling the polymer structure is key to making specialty polymers that have very specific features.
In recent research published in the Journal of the American Chemical Society(opens in new window), chemistry doctoral student Xiaolei Hu developed a new method — light-driven miniemulsion atom transfer radical polymerization (ATRP) — that provides precise control over the resulting polymer structure. The novel method expands the scope of polymers that can be created using emulsion polymerization.
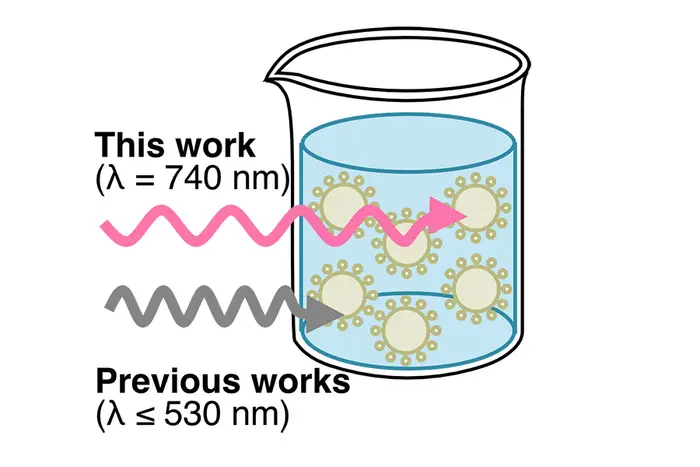
“This is the first example of robust and efficient miniemulsion photoATRP under long- wavelength light, particularly red and near infrared lights,” said Krzysztof Matyjaszewski(opens in new window), J.C. Warner University Professor of Natural Sciences. “Further exploration and application of this technique in industrial settings could lead to the sustainable and economic production of well-defined/specialty polymers.”
ATRP, the most robust method of controlled polymerization, allows scientists to string together monomers in a piece-by-piece fashion. By having precise control of polymer growth and shape, scientists can create highly tailored polymers with specific properties. However, running a successful ATRP reaction in an emulsion presents a variety of challenges, many of which have been overcome through work in the Matyjaszewski group. Hu, a third-year doctoral student advised by Matyjaszewski, leverages photo-induced ATRP under longer-wavelength light so that it could be used more effectively in an emulsion.
Classically, emulsion polymerizations are triggered by adding a chemical reagent, explained Hu, but he had a different goal. He aimed to develop a greener ATRP reaction that can run under environmentally benign reaction conditions, such as in water, in open air and that can be initiated by a light source.
"These days, photochemistry — essentially using low-energy light to initiate and mediate the polymerization — has attracted great attention because of its easy setup and its green nature," Hu said.
While other researchers have used light to initiate an emulsion polymerization, they’ve used ultraviolet or short-wavelength visible light, which scatters when it shines into an emulsion, which is essentially a mixture of liquids that do not dissolve in each other such as oil in water. Because the light scatters, it isn’t uniformly available throughout the emulsion to trigger polymerization, resulting in slow reactions with limited control over the final polymer properties. This was especially true in large-scale reactions.
Chemistry doctoral student Xiaolei Hu
To circumvent this issue, Hu turned to longer wavelengths of light in the red and near-infrared regions of the spectrum. He used a common dye, methylene blue, as a photocatalyst. Photocatalysts are materials that activate when exposed to light, thus driving the chemical reaction. Methylene blue is a commercially available, water-soluble dye that is used in many applications, including as a medication.
In an earlier paper published in JACS, Hu found that this dye has very interesting photochemical properties that were applicable to ATRP. He demonstrated that methylene blue performed exceptionally well in a homogeneous solution in the open air, leading to rapid and well-controlled polymerization.
“We were really motivated by this result, so we worked to develop a photoATRP system for emulsions using methylene blue as the photocatalyst under red/near-infrared light,” Hu said.
In the current work, Hu found that red/near-infrared light led to enhanced polymerization in a miniemulsion while UV light resulted in almost no polymerization. The scientists also showed that methylene blue, when exposed to red/near-infrared light, induces the rapid initiation of the ATRP process and provides control over polymerization.
Most important, according to Hu, is the discovery that the longer wavelengths of light used in the new approach maintained efficient polymerization even in larger reactors. “This finding is crucial for the practical application of photoinduced emulsion polymerization in a commercial setting,” Hu said.
Along with Hu and Matyjaszewski, graduate student Rongguan Yin and chemistry alumnus Jaepil Jeong are authors of the JACS paper. Funding for the research includes support from the National Science Foundation (CHE 2000391) and the Department of Energy (DE-SC0018784).